CASES WORLDWIDE
The Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an ongoing global health emergency.
The spread of SARS-CoV-2 was officially defined as a pandemic by the World Health Organization (WHO) on March 11, 2020 due to its sudden emergence and expansion around the world.
As of 25th October 2020, there are 10 Vaccine candidates in the late stage of clinical trials, with expected clinical readouts by the end of 2020, or early 2021.
Types of Viral Protein and Detection Methods
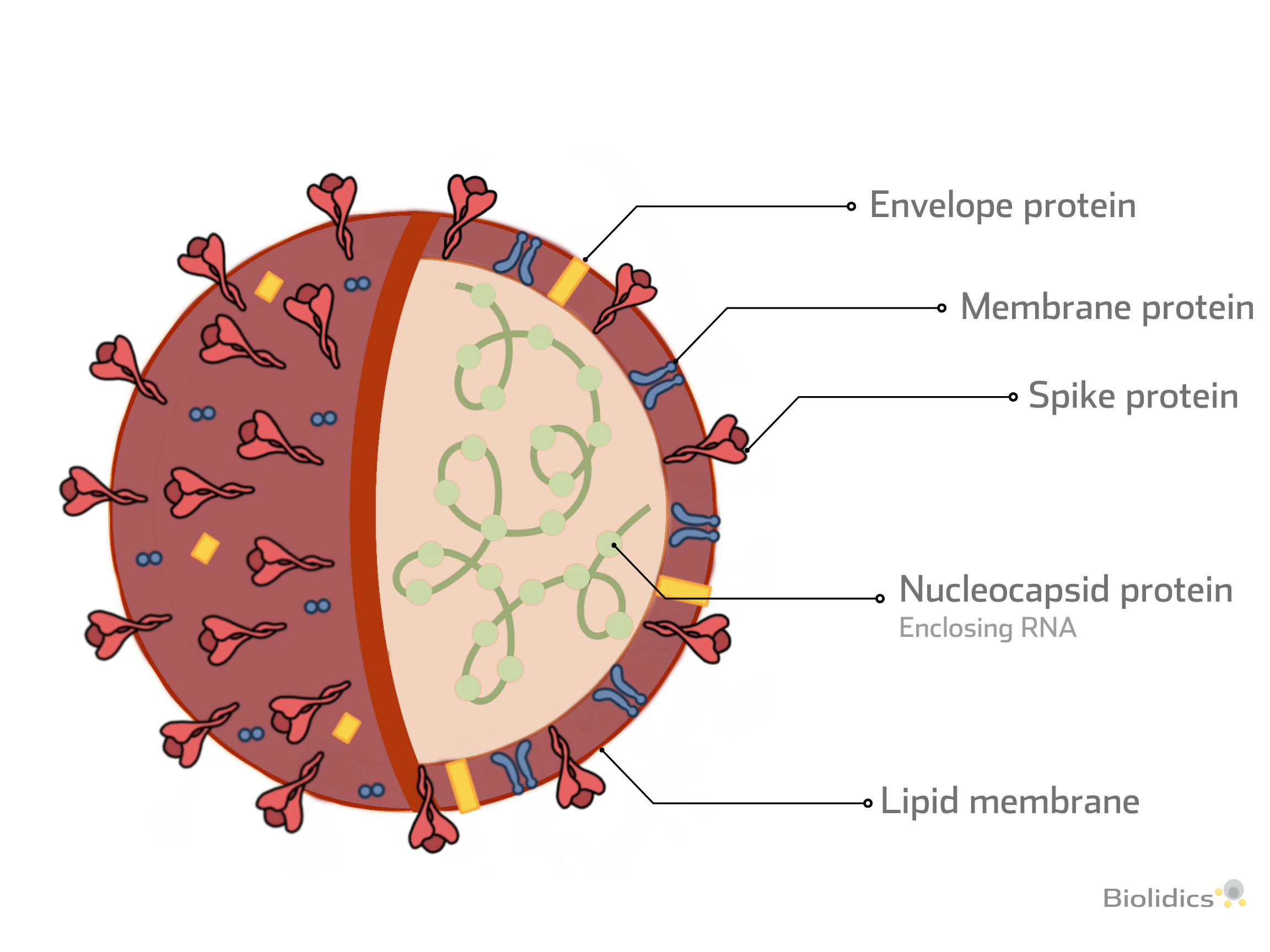
-
Spike protein (S)
- Structural proteins required for attachment to host proteins
-
Nucleocapsid (N)
- N protein is conserved and abundant, making it an ideal detection marker for SARS-CoV-2
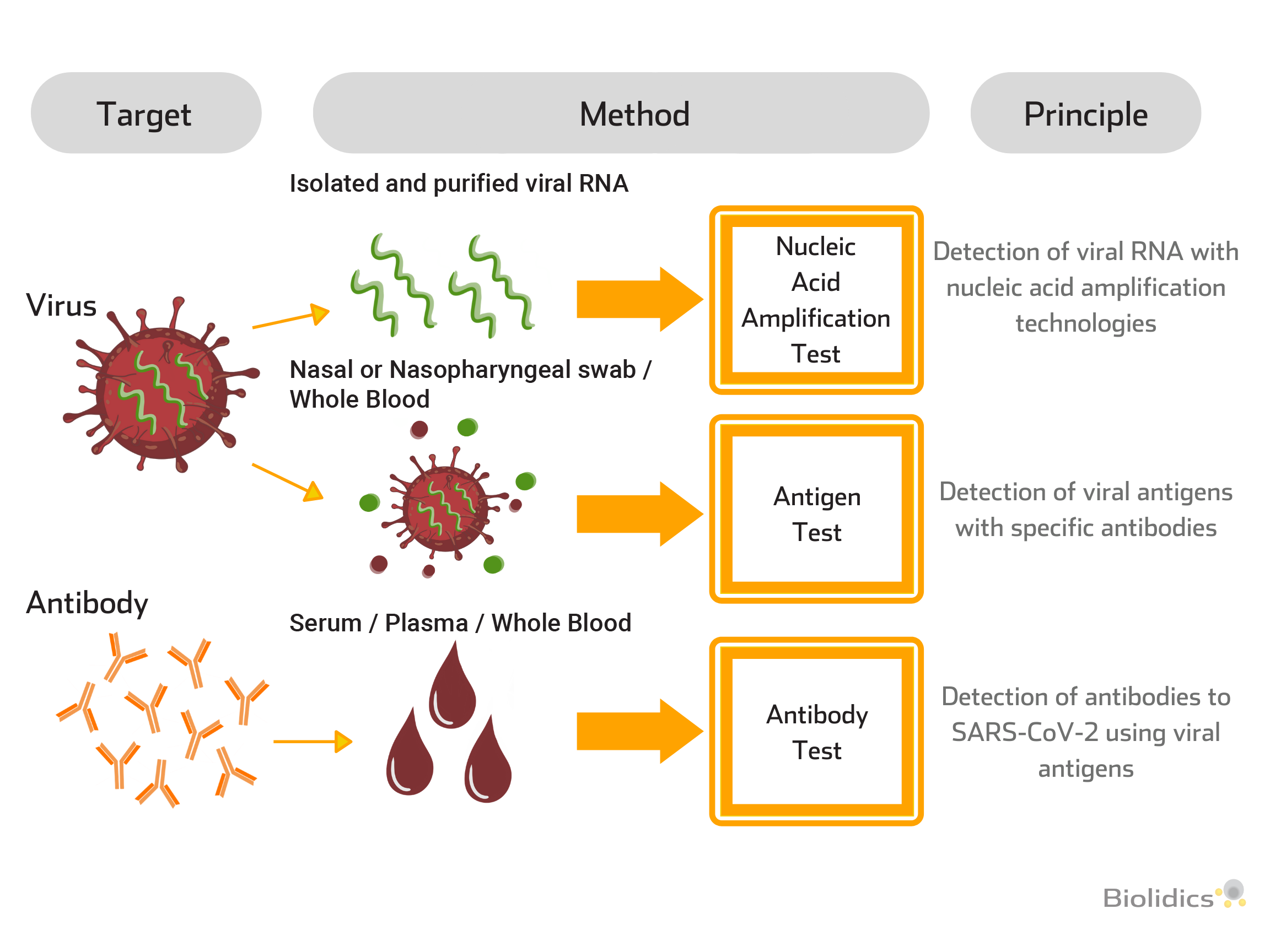
SARS-CoV-2 Testing Applications at Different Infection Stages
A combination of blood-based and swab methods allows for the cross-verifications of different test methods at different stages of the disease.
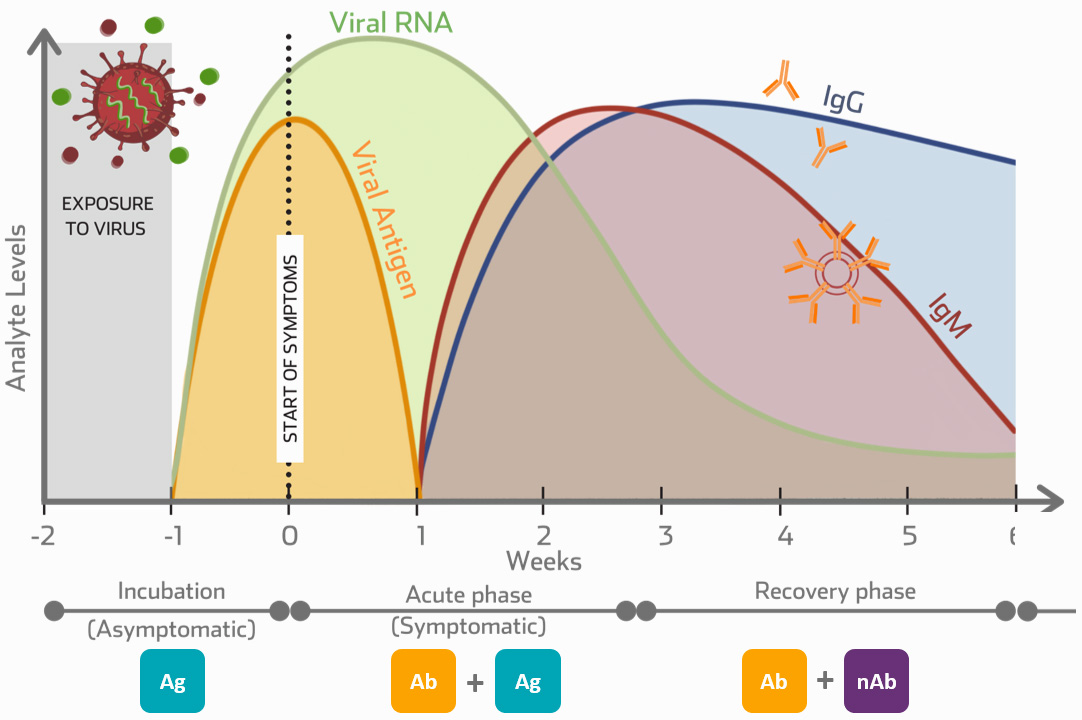
|
Ab
|
IgM/IgG Antibody Testing
Pre-vaccination insights and applications |
Ag |
Antigen Testing
Antigen tests have a faster turnaround time in comparison to PCR tests, making antigen testing more suitable as a bedside testing tool |
nAb |
Neutralising Antibody Testing
Post-vaccination insights and applications |
Vaccine Applications
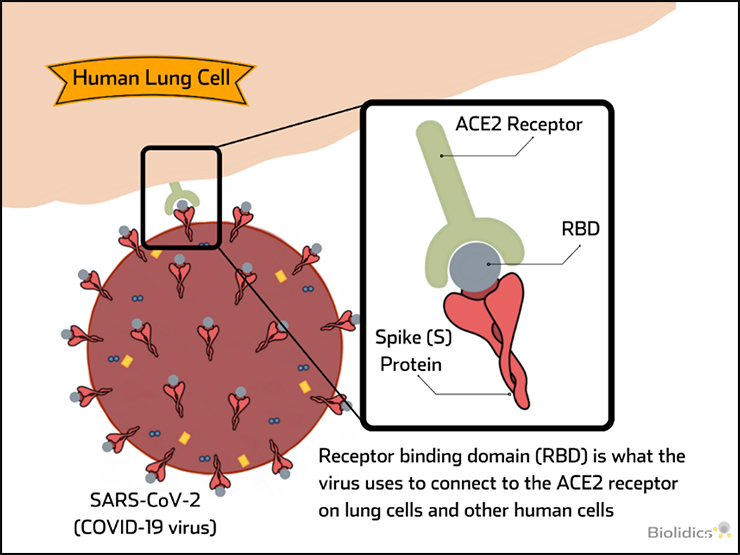
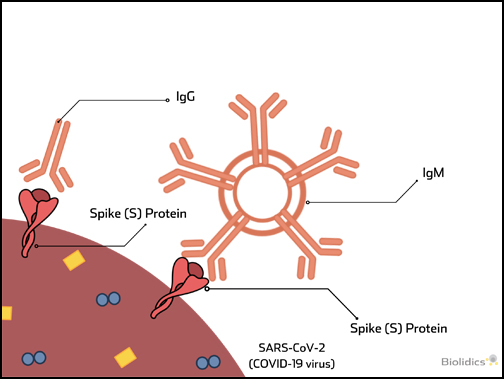
Pre-vaccination detection of IgG / IgM antibodies for SARS-CoV-2
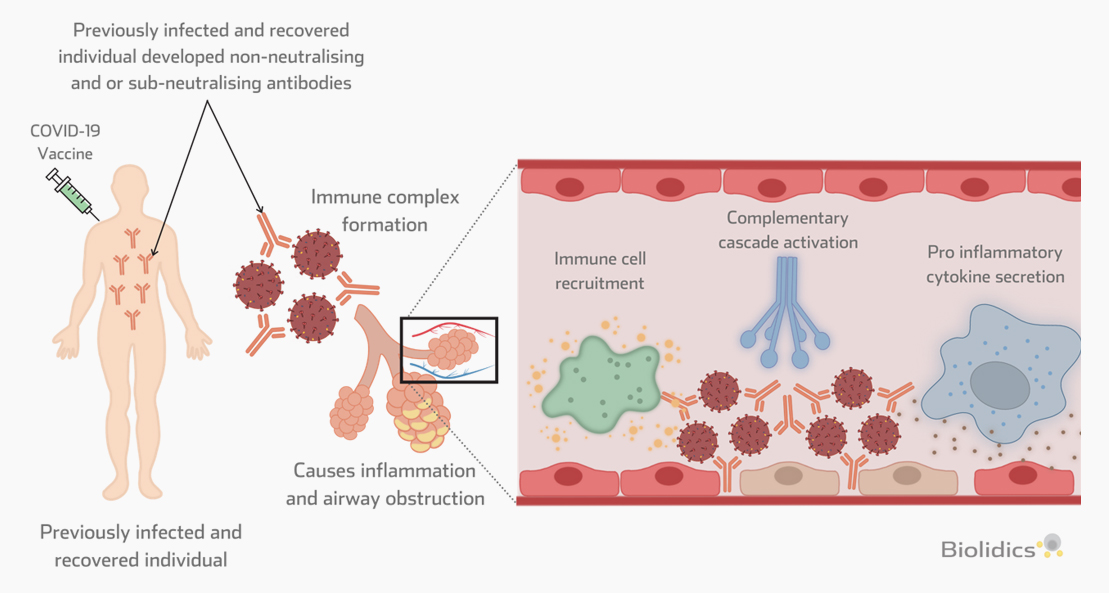
Patients with existing antibodies against SARS-CoV-2 that are administered a COVID-19 vaccine are at risk for Vaccine-associated disease enhancement (VADE) which can induce adverse effects including cytokine storm and death, as described in the figure below.
Therefore pre-vaccination screening for COVID-19 IgG/IgM antibodies is an important safety measure.
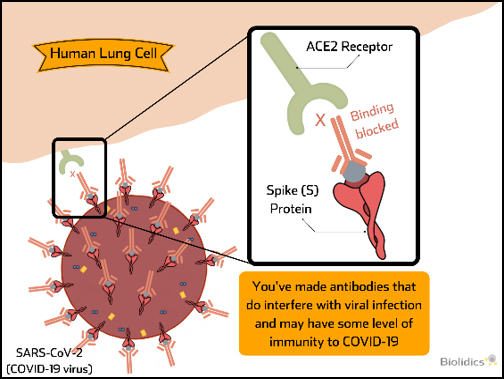
Post-vaccination detection of SARS-CoV-2 Neutralising Antibodies (nAb)
nAb can block virus binding and provide immunity to virus upon exposure and their presence is indicative of successful vaccination. nAb tests can be used to determine the presence of these antibodies following vaccination and periods of time after to confirm persistence of nAb levels over time.
Therefore nAb tests have value in monitoring to indicate successful vaccination and when repeated vaccination may be required.
Vaccine-associated disease enhancement (VADE)
The Products
| Catalogue Number | Test Type | Test Method | |
|---|---|---|---|
| CBB-F016028-BLD-C | ClearEpi® SARS-CoV-2 Antigen Rapid Test Kit | LFA | Request for a quotation or fill out our product enquiry form here |
| CBB-F016026 | Antigen Rapid Test | LFA | |
| CBB-F016031-BLC-C | Neutralising Antibody (Coming soon) | LFA / ELISA | |
| CBB-F015016-B1 | Biolidics’ 2019-nCoV IgG/IgM Antibody Detection Test | LFA |
1Tang, Y. W., Schmitz, J. E., Persing, D. H., & Stratton, C. W. (2020, May 26). Laboratory Diagnosis of COVID-19: Current Issues and Challenges. Journal of Clinical Microbiology. https://jcm.asm.org/content/58/6/e00512-20.
2Centers for Disease Control (CDC). (2020, May 8). SARS-CoV-2 (COVID-19) Fact Sheet, Guidance –Proposed Use of Point-of-Care (POC) Testing Platforms for SARS-CoV-2 (COVID-19. CDC. https://www.cdc.gov/coronavirus/2019-ncov/downloads/OASH-COVID-19-guidance-testing-platforms.pdf

