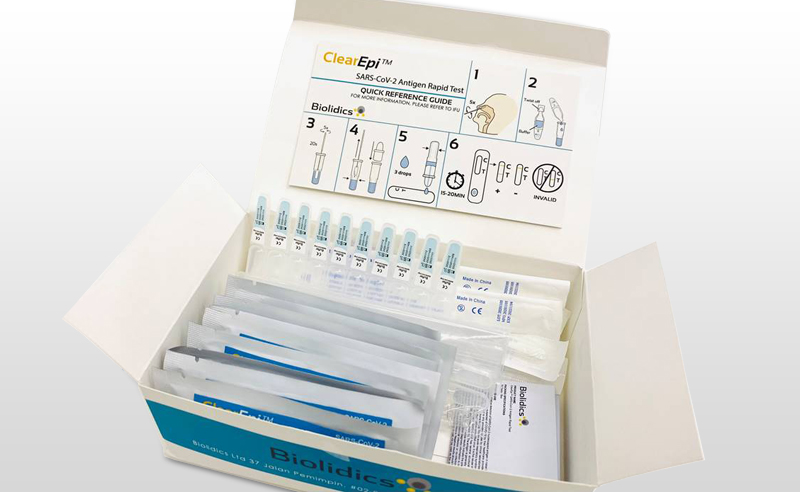
ClearEpi™ SARS-CoV-2 Antigen Rapid Test - CBB-F016028-BLD-C

- Qualitative detection of SARS-CoV-2 nucleocapsid antigen in nasal specimen directly from individuals suspected of COVID-19 infection
- Point of care testing with no additional equipment required
- Convenient storage conditions : 2 - 30°C
- High sensitivity, specificity and accuracy

(DE/CA70/40838-154686)
PEER COMPARISON
| ClearEpi™ CBB-F016028-BLC-C | Product A | Product B | Product C | Product D | Product E | |
|---|---|---|---|---|---|---|
| Sample Type | Nasal swab | Nasopharyngeal Swab | Nasopharyngeal / Throat Swab | Nasal swab | Nasopharyngeal swab | Nasal or Throat swabs |
| LOD | 1.6 x 102 TCID50/mL | 2.5 x 101.8 TCID50/mL | 3.06 x 102.2 TCID50/mL | NA | NA | 4.25 x102 TCID50/mL |
| Clinical Sensitivity | 98.15% | 91.4% | 76.6% | 86.44% | 97.5% | 90.09% |
| Clinical Specificity | 98.75% | 99.8% | 99.3% | 100% | 100% | 97.52% |
| Accuracy | 98.60% | 97.8% | 98.41% | 94.44% | 99.0% | 95.24% |
| Read time | 15~20 mins | 15~30 mins | 15~30 mins | 15~20 mins | 10~15 mins | 15~20 mins |
| Certification | CE marked | FDA EUA | CE marked WHO EUL | CE marked, NMPA | CE marked | CE marked |
TEST PROCEDURE AND INTERPRETATION

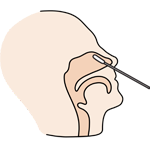



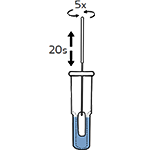

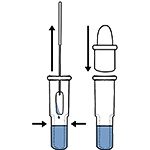

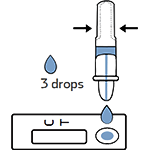
Tear off the foil pouch, take out the test strip/cassette and place the test kit on a clean and leveled surface. Label the test device and one extraction tube for each specimen or control to be tested. Gently squeeze the ridged body of the tube, dispensing (3) drops of the processed specimen into the sample well.

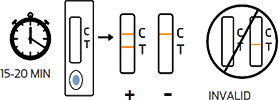
Read the test results between 15 and 20 minutes.
* Do not read the results after 20 minutes

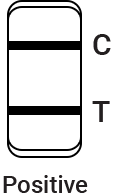



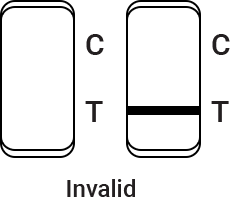
Control line fails to appear. Insufficient buffer volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the procedure with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.

The result should be judged within 15~20 minutes after the sample is added into the sample well, and the result displayed after 20 minutes is invalid.
CLINICAL DATA PERFORMANCE
The kit showed 98.15% of sensitivity and 98.75% of specificity.
| Reagent Test Results | RT-PCR Comparator | Subtotal | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 53 | 2 | 55 |
| Negative | 1 | 158 | 159 |
| Subtotal | 54 | 160 | 214 |
Positive Percent Agreement (PPA) = 53/54 (98.15%) (95%CI: 90.1%~100.0%)
Negative Percent Agreement (NPA) = 158/160 (98.75%) (95%CI: 95.6%~99.8%)
Accuracy = (53+158)/214×100% = 98.60%
INTENDED USE
For in vitro qualitative detection of SARS-CoV-2 nucleocapsid antigen in nasal(NS) swab specimens directly from individuals who are suspected of COVID-19 by their healthcare provider within the first 5 days after onset of symptoms.
This test is only provided for use by clinical laboratories or to healthcare workers for point-of-care testing, not for at-home testing.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, or 2019-nCoV) is an enveloped non-segmented positive-sense RNA virus. It is the cause of coronavirus disease 2019 (COVID-19), which is contagious in humans. SARS-CoV-2 has several structural proteins including spike (S), envelope (E), membrane (M), and nucleocapsid (N).
The antigen is generally detectable in upper respiratory samples during the acute phase of infection. Positive results indicate the presence of viral antigens, but the clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out a bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Negative results should be treated as presumptive, which do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management. For in vitro diagnostic use only. For professional use only.
LIMITATIONS
This product is only suitable for a qualitative test and auxiliary diagnosis. The test results are only for clinical reference and should not be the only basis for clinical diagnosis and treatment.
The clinical management of patients should be considered in combination with their symptoms, physical signs, medical history, other laboratory tests, therapeutic reaction, and epidemiological information. Users should test specimens as quickly as possible after specimen collection. Positive test results do not rule out co-infections with other pathogens.
Results from the test should be correlated with the clinical history, Epidemiological data, and other data available to the clinician evaluating the patient. A false-negative test result may occur if the level of viral antigen in a sample is below the detection limit of the test or if the sample was collected or transported improperly; therefore, a negative test result does not eliminate the possibility of SARS-CoV-2 infection. The amount of antigen in a sample may decrease as the duration of illness increases.
Specimens collected after day 5 of illness are more likely to be negative compared to an RT-PCR assay. Failure to follow the test procedure may adversely affect test performance and/or invalidate the test result. The contents of this kit are to be used for the qualitative detection of SARS-CoV-2 antigens from nasal swab specimens only.
The kit performance depends on antigen load and may not correlate with other diagnostic methods performed on the same specimen. Negative test results are not intended to rule in other non-SARS- CoV-2 viral or bacterial infections. Positive and negative predictive values are highly dependent on prevalence rates. Positive test results are more likely to represent false-positive results during periods of little/no SARS-CoV-2 activity when disease prevalence is low. False-negative test results are more likely when the prevalence of disease caused by SARS-CoV-2 is high.
This kit has been evaluated for use with human specimen material only. Monoclonal antibodies may fail to detect or detect with less sensitivity, SARS-CoV-2 viruses that have undergone minor amino acid changes in the target epitope region.
The performance of this test has not been evaluated for use in patients without signs and symptoms of respiratory infection and performance may differ in asymptomatic individuals. The sensitivity of the test after the first five days of the onset of symptoms has been demonstrated to decrease as compared to an RT-PCR SARS-CoV-2 assay. Negative results should be treated as presumptive and confirmed with an FDA authorized molecular assay, if necessary, for clinical management, including infection control.
Specimen stability recommendations are based upon stability data from influenza testing and performance may be different from SARS-CoV-2. Users should test specimens as quickly as possible after specimen collection, and within one hour after specimen collection. The validity of the kit has not been proven for identification /confirmation of tissue culture isolates and should not be used in this capacity.