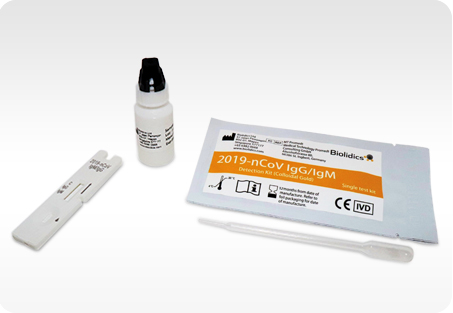
2019-nCoV IgG/IgM Antibody Detection Kit
(Colloidal Gold)

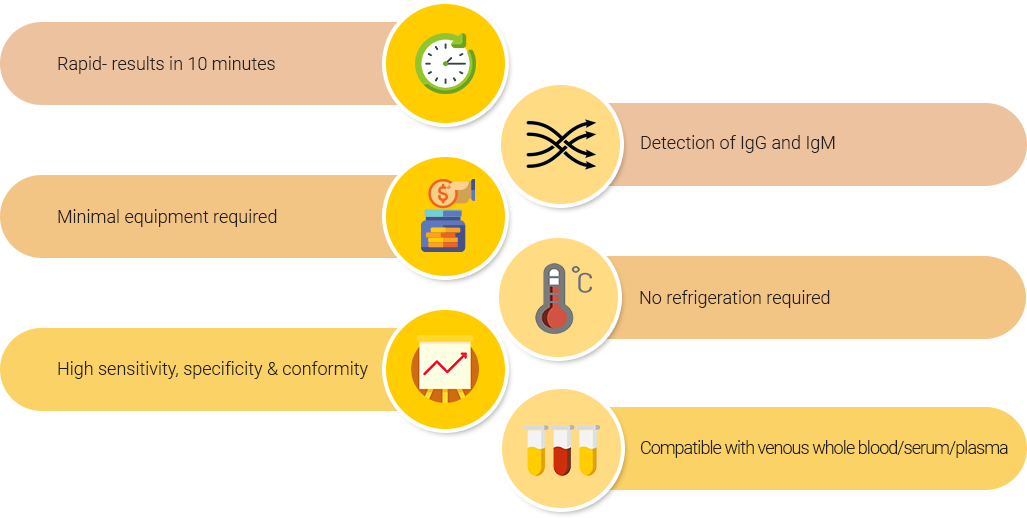
- High sensitivity, specificity & conformity
- Compatible with serum/plasma/venous whole blood1
- Simultaneous detection of SARS-CoV-2 specific IgM and IgG antibodies

-
 HSA 600:36/01
HSA 600:36/01 
-
 European CE Marking
European CE Marking
(DE/CA70/40838-154686)
1 Not recommended for finger prick test. Validations were performed on venous whole blood, serum and plasma.
2 Venous whole blood and serum specimens were tested using the 2019-nCoV IgG/IgM Detection Kit and results were compared to clinical diagnosis.
OUR METHOD
COMPARISON AGAINST OTHER METHODOLOGY

| RT-PCR | Antigen Rapid Test |
IgG/IgM Rapid Test |
|
|---|---|---|---|
| Detection Substance | Nucleic Acid | Virus Antigen |
IgG/IgM antibodies against virus |
| Sample Type | Nasopharyngeal Swabs/ Sputum/ Alveolar/ Lavage Fluid | Nasopharyngeal Swabs/ Sputum |
Serum/Plasma/ Venous Whole Blood |
| Result Time | 2 hours | 10-15 minutes | 10 minutes |
| Instrument(s) Required | Required | Not Required | Not required |
| Laboratory Requirements | High | Average | Average |
| Product Usage and comments | Confirming Diagnosis | Diagnostic Support Tool alongside epidemiology checks (temperature checks, health questionaires etc) | |
| The 2019-nCov IgG/IgM Rapid Test Device is a rapid chromatographic immunoassay for the qualitative detection of IgG & IgM antibody of Coronavirus in human venous whole blood, serum or plasma. | |||
PEER COMPARISON
| BIOLIDICS | Product A | Product B | Product C | Product D | Product E | |
|---|---|---|---|---|---|---|
| Time to Results | 10 minutes | 10 minutes | 10 minutes | 10 minutes | 10 minutes | 10 minutes |
| Sample Types | Serum / Plasma / Venous Whole blood | Serum / Plasma / Venous Whole Blood | Serum / Plasma / Venous Whole Blood | Serum / Plasma / Venous Whole Blood | Serum / Plasma / Venous Whole Blood | Serum / Plasma / Venous Whole Blood |
| Clinical Sensitivity | 91.54% | 90.2% | 87.3% | >90% | 86.43% | 97.9% |
| Clinical Specificity | 97.02% | 99.2% | 100% | >90% | 99.57% | 91.77% |
| Conformity | 95.09% | 95.2% | Not Available | Not Available | 91.61% | Not Available |
| Certifications | CE Marking submission/Health Science Authority (SG)/Philippines FDA | NMPA/CE marking | NMPA/CE marking | CE Marking/FDA-EUA submission | NMPA/CE marking | FDA-EUA submission |
TEST PROCEDURE AND INTERPRETATION
| Intended Samples for Testing | Human Serum | Plasma | Venous Whole Blood |
|---|---|---|---|
| Collection Tube |
Plain tube with or without gel (5mL)
|
Heparin Sodium or EDTA Anticoagulant blood tube (3mL)
|

EDTA Anticoagulant blood tube (3mL)
|
| Storage Conditions |
Serum/plasma stored at 2-8°C ; 7 days minus 20°C(frozen) and below; 24 days |
Venous whole blood must be tested immediately | |
| Other Important Notes |
|
||
Haemolysed or hyperlipidaemic blood samples should not be tested using our 2019-nCoV IgG/IgM Antibody Detection Kit.
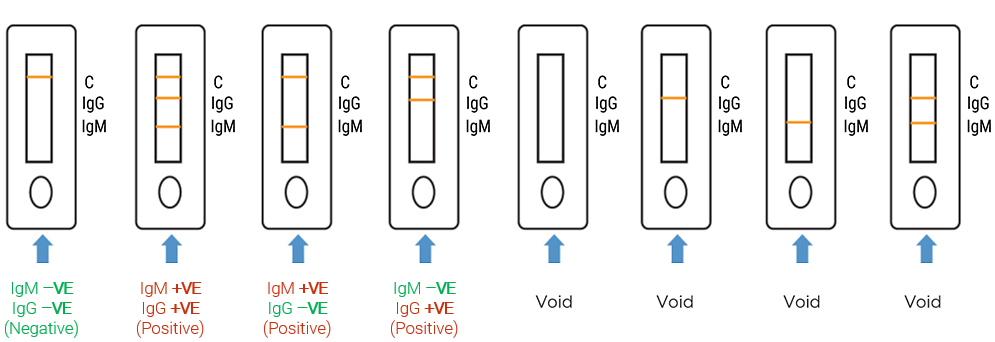
| Results* | Interpretation** |
|---|---|
| IgM +VE, IgG +VE | Suspected recent infection of SARS-CoV-2 |
| IgM +VE, IgG –VE | Suspected recent infection of SARS-CoV-2 |
| IgM –VE, IgG +VE | Patient suspected to have past infection |
| IgM –VE, IgG –VE | IgG/IgM antibodies for SARS-CoV-2 undetected OR antibody level below limit of detection |
* Results should only be evaluated if the C band is present. If it is absent, the test must not be evaluated and has to be discarded. A retest of the sample will be required.
** This test is intended to be an aid in identifying patients with antibodies to SARS-CoV-2. Positive IgG and/or IgM results indicates a recent or prior infection. This test is not intended for use to diagnose acute SARS-CoV-2 infection and direct testing should be performed for diagnosis.
FREQUENTLY ASKED QUESTIONS
-
What are the different types of tests and how are they different?
There are two main types of tests to detect the SARS-CoV-2 virus: Molecular and Serological. A molecular test detects the RNA, which is the virus’ genetic material. A serological test detects proteins, called antibodies, that are produced by the immune system in response to an infection.
-
When do antibodies to SARS-CoV-2 surface?
Upon an infection, the production of IgM and IgG by the human body occurs at different time points. The IgM antibodies are usually produced in the early to mid-stages of an initial infection, whereas, the IgG antibodies are present in the mid to late-stages of an infection, and after recovery as well.
-
Who should perform the test?
Only professionally trained operators are required to carry out the test.
-
Where can this test be carried out?
In primary care clinics, hospitals, or other designated healthcare facilities. It is not intended for testing outside of healthcare settings, such as home.
-
Can I purchase a test for my personal use at home?
No. Only professionally trained operators can carry out the test under approved medical settings.
-
What is the difference between Sensitivity, Specificity and Accuracy?
The terms "Sensitivity", "Specificity" and "Accuracy" refer to the different statistical parameters used in describing the performance of a test. The accuracy of a medical test is determined by the sensitivity and specificity of the test.
Sensitivity measures the number of patients, who have developed the antibodies against SARS-CoV-2, have been correctly identified as a patient infected with SARS-CoV-2 (also known as “true positive” rate).
Specificity measures the number of patients, who have not developed the antibodies against SARS-CoV-2, have been correctly identified as an uninfected patient of SARS-COV-2 (also known as “true negative” rate).
Accuracy, or Conformity, is the proportion of true results, either true positive or true negative, in a group of patients who has been tested
INTENDED USE
The 2019-nCoV IgG/IgM Antibody Detection Kit (Colloidal Gold) is intended for the qualitative detection of IgG/IgM antibodies against SARS-CoV-2 in human serum, plasma, and venous whole blood collected in clinical laboratories and/or by healthcare workers at the point-of-care.
The results from this test should not be used as the sole basis to diagnose, exclude SARS-CoV-2 infection and/or to inform infection status. The results will have to be interpreted together with clinical presentation and are to be confirmed with supplemental testing (e.g. RT-PCR).
The kit is not intended for finger-prick testing, in-home testing or screening donated blood.
LIMITATIONS
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out other infections in these individuals.
- Results from antibody testing should not be used to diagnose or exclude acute SARS-CoV-2 infection.
- This test has been authorized only for the presence of IgM and IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens



